v/t gas law|pressure and temperature relationship thermodynamics : purchase Charles' law, or the law of volumes, was founded in 1787 by Jacques Charles. It states that, for a given mass of an ideal gas at constant pressure, the volume is directly proportional to its absolute temperature, assuming in a closed system. The statement of Charles' law is as follows: the volume (V) of a given mass of a gas, at constant pressure (P), is directly proportional to its temperature (T). Resultado da Probo Apk Download – Trusted Opinion Trading App. Probo apk is the best possible opinion trading app in India to earn quick money online. On every .
{plog:ftitle_list}
Video quality All; 720P + 1080P+ Milf le gusta el creampie 3 .
who discovered ideal gas law
Standard Launder Tester distribute
who created ideal gas law
Boyle’s Law describes the inverse proportional relationship between pressure and volume at a constant temperature and a fixed amount of gas. This law came from a manipulation of the Ideal Gas Law. \[ P \propto \dfrac{1}{V} \] or expressed from two pressure/volume points: .You have a fixed mass of gas, so n (the number of moles) is constant. R is .The number of molecules or atoms in a specific volume of ideal gas is .
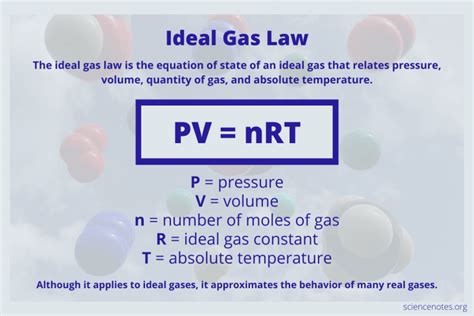
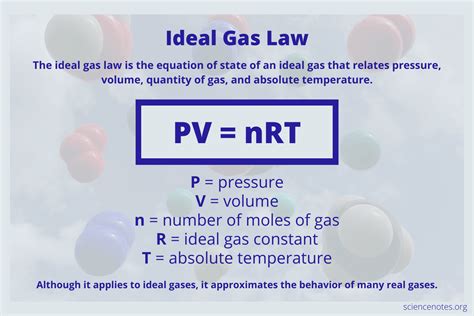
The ideal gas law, also called the general gas equation, is the equation of state of a hypothetical ideal gas. It is a good approximation of the behavior of many gases under many conditions, although it has several limitations. It was first stated by Benoît Paul Émile Clapeyron in 1834 as a combination of the empirical Boyle's law, Charles's law, Avogadro's law, and Gay-Lussac's law. The ideal ga.Charles' law, or the law of volumes, was founded in 1787 by Jacques Charles. It states that, for a given mass of an ideal gas at constant pressure, the volume is directly proportional to its absolute temperature, assuming in a closed system. The statement of Charles' law is as follows: the volume (V) of a given mass of a gas, at constant pressure (P), is directly proportional to its temperature (T). The ideal gas law allows us to calculate the value of the fourth variable for a gaseous sample if we know the values of any three of the four variables (P, V, T, and n). It .
Ideal gas law, relation between the pressure P, volume V, and temperature T of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move almost independently of each other. In . Charles’s law or the law of volumes is an ideal gas law that states that the volume and temperature of a fixed amount of gas are proportional at constant pressure. Doubling the temperature of a gas doubles its volume. .
Perspiration Color Fastness Tester distribute
gas laws, laws that relate the pressure, volume, and temperature of a gas. Boyle’s law —named for Robert Boyle —states that, at constant temperature, the pressure P of a gas varies inversely with its volume V , or P .To use the ideal gas law to describe the behavior of a gas. In Section 10.3 "Relationships among Pressure, Temperature, Volume, and Amount", you learned how the volume of a gas changes . The ideal gas law allows us to calculate the value of the fourth quantity (P, V, T, or n) needed to describe a gaseous sample when the others are known and also predict the .V ∝ T (P constant) pressure-temperature (constant volume) The pressure of a gas is directly proportional to its temperature when volume is constant. The ratio of pressure to temperature .
The ideal gas law is a generalization containing both Boyle’s law and Charles’s law as special cases. This law can be derived from the kinetic theory of gases and relies on the assumptions that (1) the gas consists of a .Applying the Ideal Gas Law. The ideal gas law allows us to calculate the value of the fourth variable for a gaseous sample if we know the values of any three of the four variables (P, V, T, and n).It also allows us to predict the final state of a sample of a gas (i.e., its final temperature, pressure, volume, and amount) following any changes in conditions if the parameters (P, V, T, .V 1 /T 1 = k and V 2 /T 2 = k \(\displaystyle \frac{V_1}{T_1}\;=\;\frac{V_2}{T_2}\) at constant pressure for a fixed amount of gas. Charles’s law can be used to determine the final temperature or volume (T 2,V 2) if a change is made to the initial temperature or volume (T 1,V 1) of a fixed amount of gas at constant pressure. In calculations .
The ideal gas law is the equation of state for ideal gases that applies to many real gases. The ideal gas law is the equation of state for an ideal gas that relates pressure, volume, gas quantity, and absolute temperature.Although the law describes the behavior of an ideal gas, it approximates real gas behavior in many cases. Uses of the ideal gas law including solving .
or. P 1 V 1 = P 2 V 2 = constant. This correlation was discovered independently by Robert Boyle (1627–1691) of Ireland in 1662 and Edme Mariotte (1620–1684) of France in 1676. In Great Britain, America, Australia, the West Indies and other remnants of the British Empire it is called Boyle's law, while in Continental Europe and other places it is called Mariotte's law.
Gas Laws The content that follows is the substance of lecture 18. In this lecture we cover the Gas Laws: Charles',Boyle's,Avagadro's and Gay Lussacs as well as the Ideal and Combined Gas Laws. . (V 1 and T 1): Boyle's Law - states that the volume of a given amount of gas held at constant temperature varies inversely with the applied pressure .
T : temperature // V : volume // P : pressure // n : number of mole . what it is clearly known about the relationship between one another is : P is inversely proportional to V ( from Boyle's law : PV=constant ) V is directly proportional to T ( from Charles's law : V/T=constant )
P 1 V 1 = P 2 V 2 = constant. volume-temperature (constant pressure) The volume of a gas is directly proportional to its temperature when pressure is constant. The ratio of volume to temperature is constant when pressure is constant. This relationship is known as Charles' law or Gay-Lussac's law. a constant pressure process is said to be isobaric.Charles's Law. French physicist Jacques Charles (1746-1823) studied the effect of temperature on the volume of a gas at constant pressure. Charles's Law states that the volume of a given mass of gas varies directly with the absolute temperature of the gas when pressure is kept constant. The absolute temperature is temperature measured with the Kelvin scale. The combined gas law is an ideal gas law that states the ratio of pressure and volume to absolute temperature is a constant. The combined gas law is an ideal gas law that combines Boyle’s law, Charles’s law, and Gay-Lussac’s law.It states the the ratio between the pressure-volume product and absolute temperature of a gas is a constant. Pressure, volume, .The ideal gas law is easy to remember and apply in solving problems, as long as you use the proper values and units for the gas constant, R. Gases whose properties of P, V, and T are accurately described by the ideal gas law (or the other gas laws) are said to exhibit ideal behavior or to approximate the traits of an ideal gas.
T T T – Temperature of the gas, measured in kelvins. To find any of these values, simply enter the other ones into the ideal gas law calculator. For example, if you want to calculate the volume of 40 moles of a gas under a pressure of 1013 hPa and at .
atm V = volume; n = number of moles; T = temperature; R = gas constant; Gas Constant R. The gas constant R is a constant of units of energy per temperature increment per mole. It is also known as the universal gas constant, ideal gas constant and molar gas constant. The value of gas constant R depends on the units you are using in your calculation. Gases whose properties of P, V, and T are accurately described by the ideal gas law (or the other gas laws) are said to exhibit ideal behavior or to approximate the traits of an ideal gas. An ideal gas is a hypothetical .
Mathematically charles law can be expressed as: V∝ T (where P and n are constant) or V = kT (where k is a constant) or V/T = k. If V 1 = the initial volume of a gas, T 1 = the initial temperature of that gas. And V 2 = the final volume of a gas, T 2 = the final temperature of that gas. The combined gas lawThis relationship between temperature and pressure is observed for any sample of gas confined to a constant volume. An example of experimental pressure-temperature data is shown for a sample of air under these conditions in .
An ideal gas is a theoretical gas composed of many randomly moving point particles that do not interact except when they collide elastically. The ideal gas law is the equation of state of an ideal gas. It relates the state variables of the gas: pressure \((P),\) volume \((V),\) and temperature \((T).\) Also included are the amount of the gas \((n)\) and the ideal gas constant \((R=8.314 .
Applying the Ideal Gas Law. The ideal gas law allows us to calculate the value of the fourth variable for a gaseous sample if we know the values of any three of the four variables (P, V, T, and n).It also allows us to predict the final state of a sample of a gas (i.e., its final temperature, pressure, volume, and amount) following any changes in conditions if the .
The Ideal Gas Law was first written in 1834 by Emil Clapeyron. What follows is just one way to "derive" the Ideal Gas Law. For a static sample of gas, we can write each of the six gas laws as follows: PV = k 1 V / T = k 2 P / T = k 3 V / n = k 4 P / n = k 5 1 / nT = 1 / k 6. Note that the last law is written in reciprocal form.
The behaviour of a Gas can be studied by various laws known as the Gas laws. Let us see more! During high pressure or high-temperature conditions, a tyre inflated with air is at the risk of bursting. With changing physical conditions the behaviour of gaseous particles also deviates from their normal behaviour. The behaviour of a Gas can be . We can modify this equation as we modified Boyle's law: the initial conditions V 1 and T 1 have a certain value, and the value must be the same when the conditions of the gas are changed to some new conditions V 2 and T 2, as long as pressure and the amount of the gas remain constant. Thus, we have another gas law:Pump gas molecules to a box and see what happens as you change the volume, add or remove heat, and more. Measure the temperature and pressure, and discover how the properties of the gas vary in relation to each other. Examine kinetic energy and speed histograms for light and heavy particles. Explore diffusion and determine how concentration, temperature, mass, and .
The equations describing these laws are special cases of the ideal gas law, PV = nRT, where P is the pressure of the gas, V is its volume, n is the number of moles of the gas, T is its kelvin temperature, and R is the ideal (universal) gas constant. n is the number of moles of the gas (mol), R is the ideal gas constant (8.314 J/(K The equations describing these laws are special cases of the ideal gas law, PV = nRT, where P is the pressure of the gas, V is its volume, n is the number of moles of the gas, T is its kelvin temperature, and R is the ideal (universal) gas constant.V T. This relationship between the temperature and volume of a gas, which became known as Charles' law, provides an explanation of how hot-air balloons work. Ever since the third century B.C., it has been known that an object floats when it weighs less than the fluid it displaces. . Gas law problems often ask you to predict what happens when .

Resultado da Follow PureNudism (@PureNudismCom) on Twitter to get the latest updates on naturism, nudist events, and family-friendly activities. Join the .
v/t gas law|pressure and temperature relationship thermodynamics